
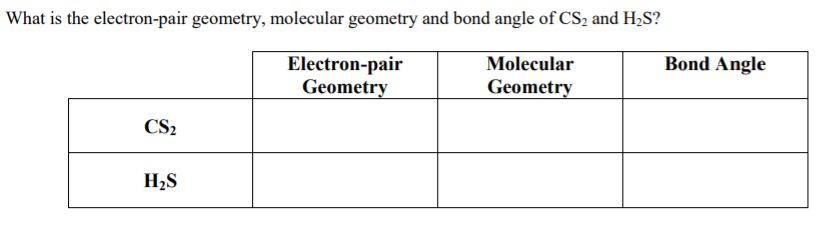
What are the different types of Covalent Bonds?īased on the number of shared electron pairs, the covalent bond can be categorized into: The covalent bonds have various categories depending on the number of shared electrons. Thus, carbon can’t accept or lose electrons, therefore, to achieve its noble gas configuration and complete the octet, it prefers sharing electrons, thereby forming a covalent bond. Besides, the C4+ would hold just two electrons by proton, thereby making the carbon unstable. In the same way, carbon can’t donate four electrons, as it would need a huge quantity of energy to eliminate four electrons. A carbon atom can’t accept four electrons, as it would be difficult for six protons to have ten electrons, and therefore the stability of the carbon will be disturbed.Ģ. Read out the article on the covalent nature of SO2.Ĭonsidering the electronic configuration of the carbon atom, it demands to accept or donate four electrons to achieve stability, which appears unlikely as:ġ. The other way is the sharing of electrons among different atoms, as seen in H2O, SO2, and CH4. First is the sharing of electrons among similar atoms, like in the case of H2, O2, etc.Ģ. Two approaches to form covalent bondingġ. The covalent bonds are moreover named molecular bonds. However, the transfer of electrons among two atoms having comparable electronegativity will not occur from their outermost shell rather, they will share electrons to fill the valence electron shell. These bonds are mainly formed within nonmetals or among the same elements. Since we are discussing the possibility of an ionic bond or covalent bond in CS2, let us dive more into the details about an ionic and covalent bond.Ī covalent bond is produced by the equal sharing of electrons among the participating atoms.
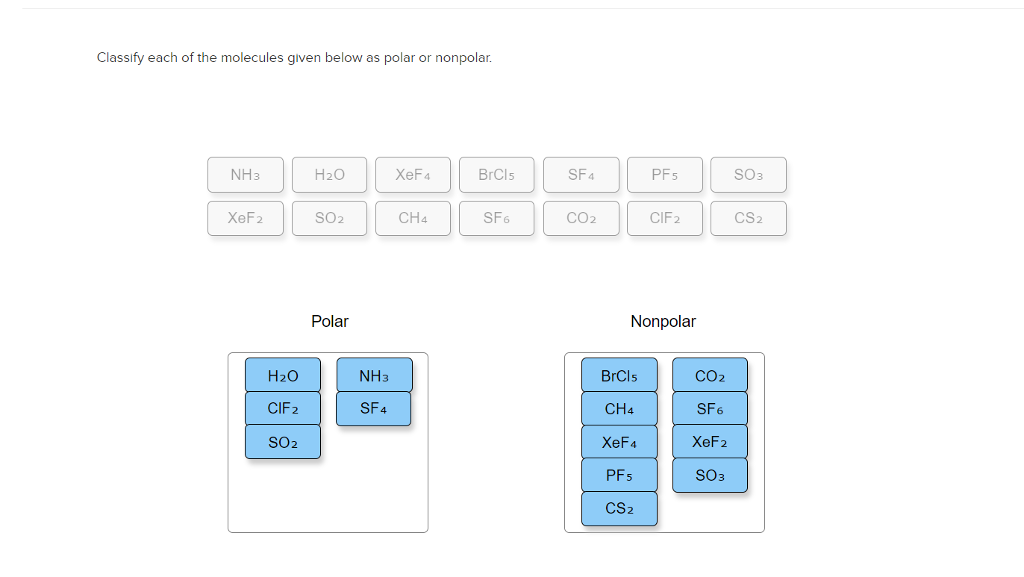
Therefore, the sharing of electrons among the participating atoms makes the bond in CS2 a covalent bond. As a result, the carbon atom shares two electrons with each sulfur atom forming a double bond. The electronegativity difference among the carbon and sulfur atom is nearly 0.03, and thus the bond between them is hardly even polar. So, is CS2 ionic or covalent? CS2 is a covalent molecule as both the carbon and sulfur atoms have nearly the same electronegativity values. So, what do you think about the bonding between the sulfur-carbon-sulfur bond? It implodes in the air and quickly catches fire. In contrast, impure carbon disulfide, typically utilized in several manufacturing purposes, is a yellowish liquid with a disagreeable odor, similar to rotten vegetables.Ĭarbon disulfide volatilizes at normal temperature, and the vapor is two times heavier as compared to air. Carbon disulfide, also known as carbon bisulfide or disulfide carbon is a chemical name for CS2, which consists of carbon and sulfide ions.Ĭarbon disulfide is a colorless liquid having a pleasing smell, somewhat similar to chloroform.


 0 kommentar(er)
0 kommentar(er)
